The atomic size of an element depends two things:
- Number of electron orbits around the nucleus
- Nuclear charge
Each of these reasons are explained below.
Number of electron orbits around the nucleus
The electrons in an atom are arranged around the nucleus in different levels called shells or orbits. For example, in a sodium (Na - 11) atom, there are a total of 11 electrons that are arranged in three successive orbits around the nucleus shown by the following diagram:
 |
| Sodium atom with three orbits |
The more the number of orbits, the lager is the atomic size. For example, potassium, which has four electron orbits, one more than sodium, has a larger atomic size than sodium:
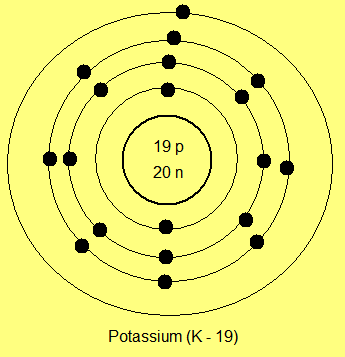 |
| Potassium atom with four orbits |
Thus we can conclude that the atomic size varies in the periodic table with the number of electron orbits. In the periodic table, the row number of an element is equal to the number of electron orbits present in its atom. Thus, as mentioned above, sodium, being in the third period, has three orbits while potassium, being in the fourth row, has four orbits.
Thus the atomic size of elements of elements increases as you move down in the groups (columns) of the periodic table.
Nuclear Charge
The atomic size of an element depends on the nuclear charge. Nuclear charge is the number of protons int the nucleus. The more the number of protons in the nucleus, more is the attractive pull on electrons around it. So out of two elements having the same number of electron orbits, the one with the greater nuclear charge has the lesser atomic size.
For example, magnesium (Mg - 12) and alumnium (Al - 13) both have the same number of orbits (since both are in the third row of the periodic table and hence both have three orbits in their atoms). But aluminium has greater atomic number (13) than magnesium (12) which means that there are more number of protons in an aluminum nucleus than in a magnesium nucleus. Thus the attractive pull between the nucleus and outer electrons in aluminum atoms is greater than in magnesium. As a result, the electron orbits in aluminum atoms move closer to the nucleus than in magnesium.
Thus two elements having the same number of orbits have different atomic sizes based on their respective nuclear charges; The one with greater nuclear charge has smaller atomic size than the one with lesser nuclear charge.
No comments:
Post a Comment